Storing renewable energy at M2N
In this theme we have two main activities: solar fuels and organic batteries.
Solar fuels
Solar-to-chemical energy conversion requires three simple steps: absorption of light, creation of charges (electrons and holes), and catalytic chemical reactions in which the charges are used to oxidize and reduce compounds in endothermic reactions such as the splitting of water and the reduction of carbon dioxide. The reaction products, e.g. hydrogen, carbon monoxide, methanol, or methane can be used directly as fuels, or used as starting products for further chemical reactions. To enable solar energy production in yields exceeding the energy conversion of natural photosynthesis (typically <1%) with cheap and abundant materials is a tremendous challenge. This has not yet been achieved in artificial systems. The crucial issue is that absorption of light, creation of charges, and at least two chemical reactions have to work in concert at the same high rate, outperforming the various recombination processes and side reactions that can occur. It has been argued that the successful construction of a direct artificial system for efficient solar fuel generation is the most important research challenge in coming years.
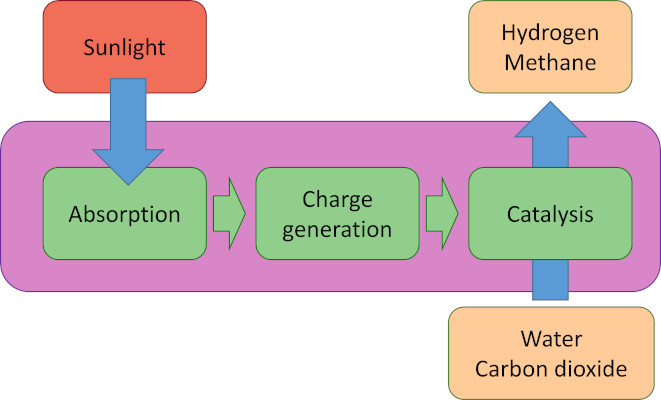 Principle of photoelectrochemical energy conversion
Principle of photoelectrochemical energy conversion
Within M2N we are working on photoelectrochemical water splitting, the oxygen evolution recation, and carbon dioxide reduction.
Water splitting
We investigate photoelectrochemical solar to hydrogen conversion using an “artificial leaf” based on organic multi-junction solar cells and catalysts for the hydrogen and oxygen evolution reactions. In 2015, we were first in developing an organic monolithic artificial leaf for unbiased water splitting with 5% solar-to-hydrogen conversion. Because a wide range of organic semiconductors is available, we can tune the maximum power point voltage of the cells to coincide with the operating point determined by the thermodynamic potential for water splitting and the overpotentials defined by the catalysts. By optimizing these parameters, a significant progress in the performance of organic artificial leaves can be achieved. With organic semiconductors we have achieved solar to hydrogen energy conversion efficiencies of about 6% in wireless and autonomous working devices. For more efficient photoelectrochemical water splitting both balancing the nature and surface area of the catalysts with the materials used in the solar cell is crucial.
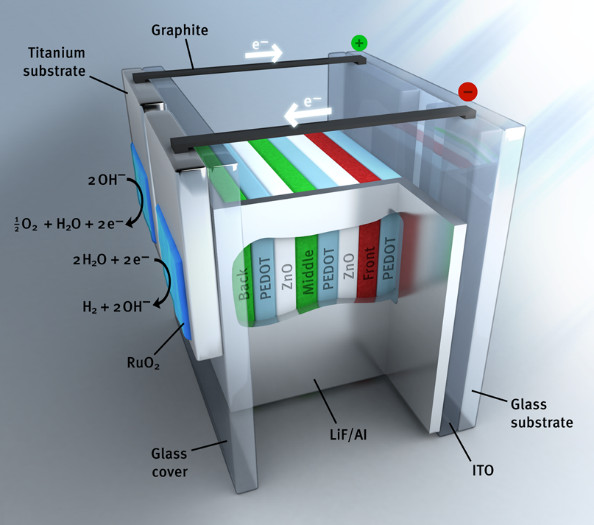 Schematic device layout of an organic photo-electrochemical cell for wireless water splitting.
Schematic device layout of an organic photo-electrochemical cell for wireless water splitting.
A recent highlight is the development of a continuous flow electrochemical reactor based on a membrane-electrode assembly which coupled to a monolithic perovskite-silicon tandem solar cell provides photo-electrochemical water splitting with a solar-to-hydrogen conversion efficiency of 21.5%. This represents a record conversion efficiency for photo-electrochemical cells operating under non-concentrated solar light.
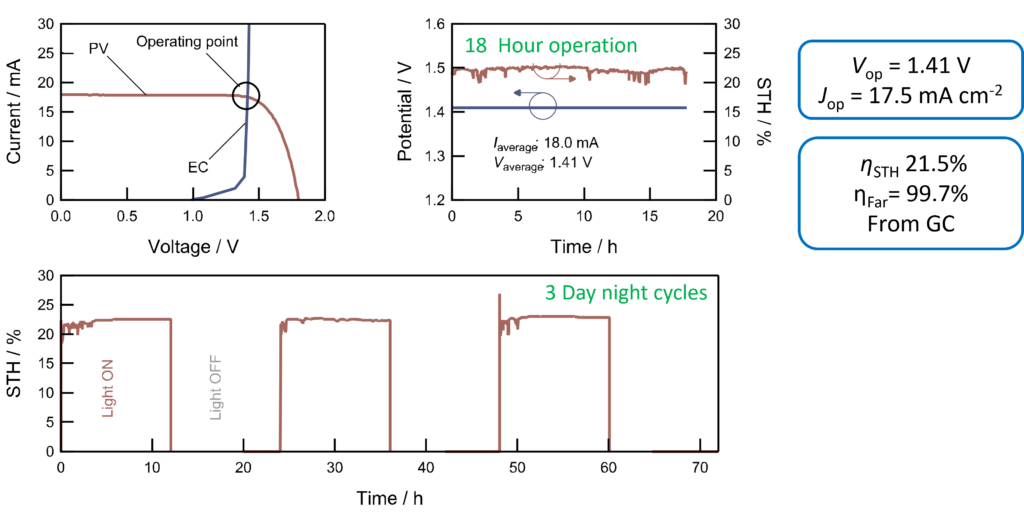
Continuous >20% solar to hydrogen water splitting at pH 7 using a perovskite-silicon tandem solar cell
Oxygen evolution reaction
Electrolysis of water is a potential source for hydrogen production on a large scale. A measure of the inefficiency in electrolysis is the so called overpotential, the voltage needed to drive the process in excess of the thermodynamic equilibrium voltage. The contribution to the overpotential of the oxygen evolution dominates overwhelmingly. A mechanism or a proper understanding for the high catalytic activity of e.g. ruthenium oxide and oxide perovskites is still unknown, but it might be that magnetic moments are at play. To this end we investigate transition metal oxide surfaces of water oxidation catalyst using spin-polarized scanning tunnelling microscopy to explore the electronic structure and the magnetic ordering of the surface layer with up to atomic resolution. Magnetic force microscopy using newly developed planar tip-on-chip probes can provide nanometer scale domain mapping as a function of applied magnetic field for correlation to oxygen evolution reaction activity.
Carbon dioxide reduction
Towards this aim of direct conversion of carbon dioxide (CO2) into fuels by light we have selected, carbon monoxide (CO) and methane (CH4) as the our targets. Also here we are working on developing new catalysts to reduce overpotentials and increase selectivity, as well as developing new photovoltaic conversion systems tailored to the electrochemical potential. We have achieved solar-to-CO energy conversion of 8-9% during continuous operation where the power was delivered by three series connected metal halide perovskite solar cells.
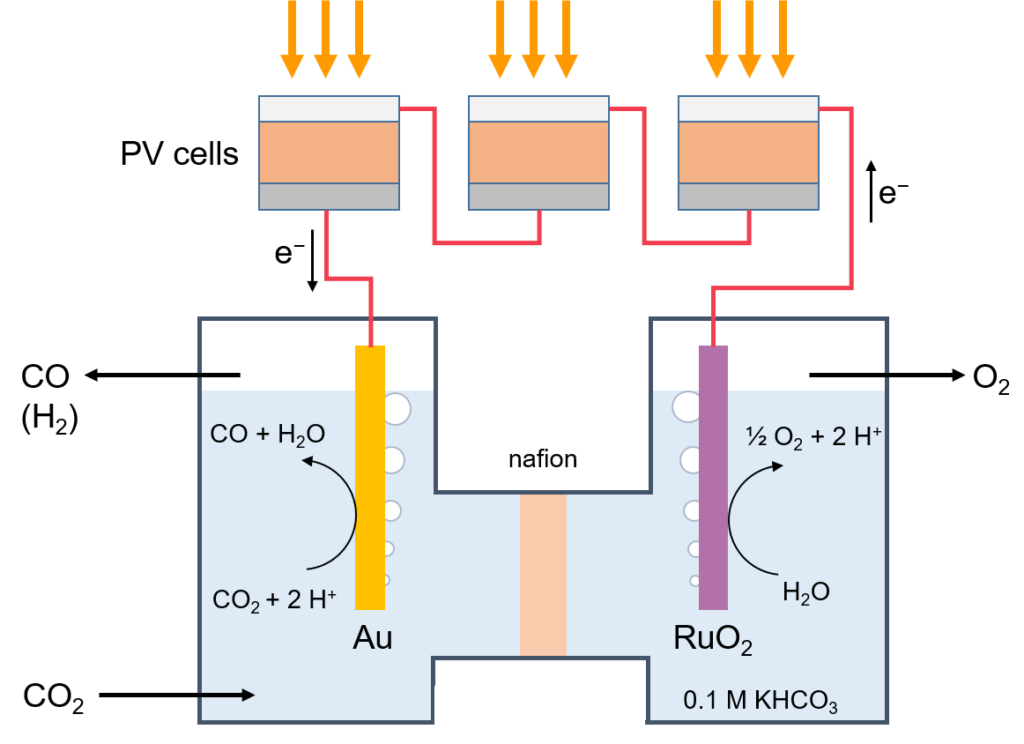 Principle of light-driven electrochemical carbon dioxide reduction.
Principle of light-driven electrochemical carbon dioxide reduction.
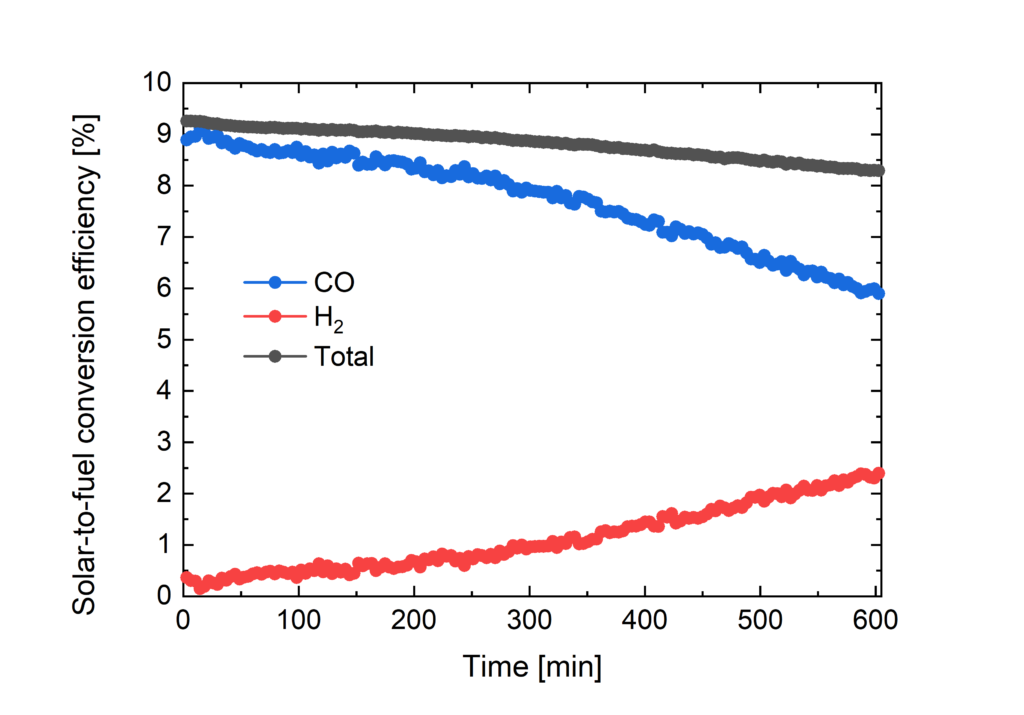 Solar-to-fuel conversion efficiencies for CO and H2 formation versus time.
Solar-to-fuel conversion efficiencies for CO and H2 formation versus time.
Contact
René Janssen
Kees Flipse
Martijn Wienk
Organic redox flow batteries
The focus is on the design, synthesis and evaluation of new redox active organic compounds that enable storing electrical energy using organic redox flow batteries. We develop stable, anolytes and catholytes and incorporate these in prototype flow batteries.
Implementation of intermittent renewable energy sources such as wind and solar in the energy grid, will require the use of large-scale energy storage to mitigate the discrepancies between energy production and demand. Non-aqueous organic redox flow batteries from abundant all-carbon based materials can provide a sustainable solution. In a redox flow battery (RFB), the redox active species are dissolved or suspended in a solvent with supporting electrolyte forming an anolyte and catholyte. These solutions flow through a reactor where they are passed over porous carbon electrodes that mediate electron transfer reactions to charge or discharge the solutions. The two half-cells in the reactor are separated by a selective permeable membrane, which allows for a compensating ion flux opposing the electrical flow of the external circuit. The advantage of this system is that the capacity of the battery is determined by the size of the electrolyte storage tanks and the power output is determined by the size of the reactor. Therefore, these metrics can be independently scaled, allowing for massive energy storage without the need of expensive large surface area electrodes. Current non-aqueous organic redox flow technology however, has not reached a mature enough level for implementation and many scientific questions still need to be answered. Specifically, new redox active materials need to be developed that simultaneously deliver high cell voltages and energy density in combination with good cyclability and stability.
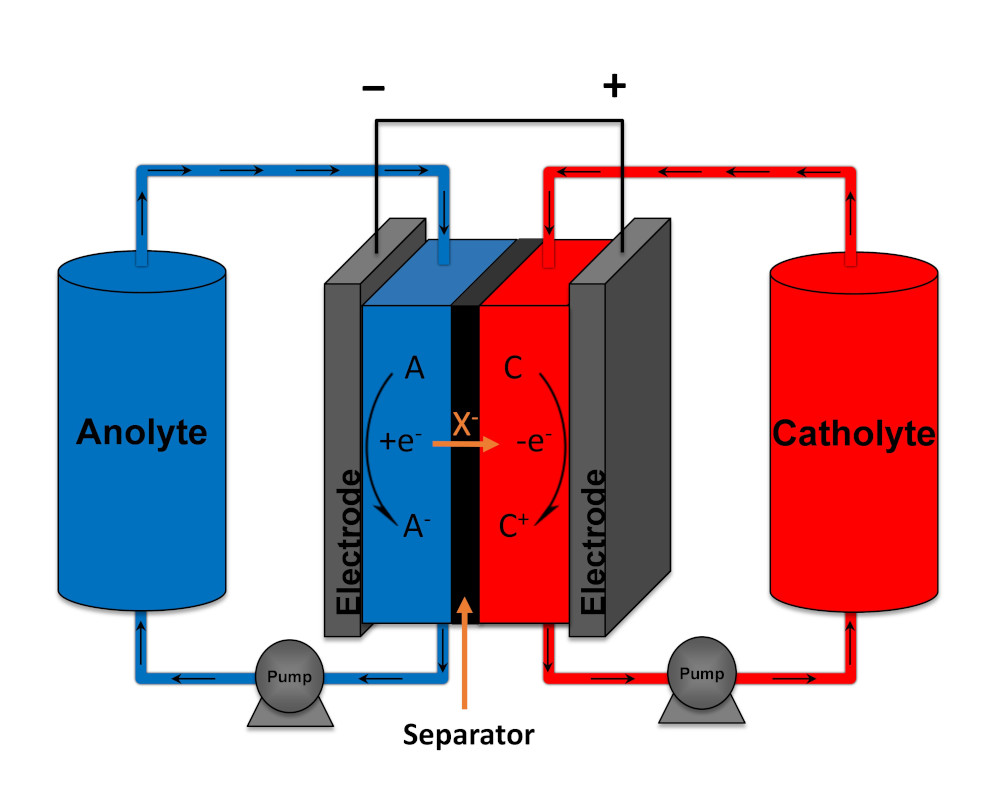
Principe of a redox flow battery
Within M2N we are working on the following aspects of organic redox flow batteries
Organic molecules for energy storage in redox flow batteries
We develop new molecules that are expected to meet the stringent requirements for this type of energy storage and advance the state-of-the-art of this technology. To discover materials that simultaneously fulfill all desired parameters such as a high redox potential, excellent solubility, and excellent (cycling) stability, we use a unique interdisciplinary approach that combines organic synthesis, electrochemistry and application in prototype devices.
Membrane evaluation
Apart from the redox active species in a RFB, the membrane separating the two half-cells is one of the crucial components in the device. Typically this element has the highest electrical resistance of the whole system and therefore has a large impact on the energy efficiency of the battery, especially at high charging or discharging rates. Its role is twofold as it must keep the anolyte and catholyte molecules separated while being highly conductive towards the supporting electrolyte. Additionally, it must have sufficient mechanical stability to withstand the shear forces of the electrolyte flow and resist swelling from the solvent. Membranes that combine all of these properties are therefore very scarce. Together with collaborators, the M2N group is developing and testing tailored membranes for this application.
Prototype flow batteries
To fully evaluate the performance of new redox active materials and membranes, a large focus lies on studying flow battery prototypes. These devices allow us to quickly evaluate the cycling stability and energy efficiencies of selected material combinations and point out what parameters need to be worked on, both on the engineering side as well as molecular design. Thanks to the straightforward scalability of flow batteries, these small prototype devices pave the way towards real world implementation of RFB storage technology.
 Orgnanic redox flow batteries in operation
Orgnanic redox flow batteries in operation
Contact
René Janssen
