Organic flow batteries at M2N
Implementation of intermittent renewable energy sources such as wind and solar in the energy grid, will require the use of large-scale energy storage to mitigate the discrepancies between energy production and demand. Non-aqueous organic redox flow batteries from abundant all-carbon based materials can provide a sustainable solution. In a redox flow battery (RFB), the redox active species are dissolved or suspended in a solvent with supporting electrolyte forming an anolyte and catholyte. These solutions flow through a reactor where they are passed over porous carbon electrodes that mediate electron transfer reactions to charge or discharge the solutions. The two half-cells in the reactor are separated by a selective permeable membrane, which allows for a compensating ion flux opposing the electrical flow of the external circuit. The advantage of this system is that the capacity of the battery is determined by the size of the electrolyte storage tanks and the power output is determined by the size of the reactor. Therefore, these metrics can be independently scaled, allowing for massive energy storage without the need of expensive large surface area electrodes. Current non-aqueous organic redox flow technology however, has not reached a mature enough level for implementation and many scientific questions still need to be answered. Specifically, new redox active materials need to be developed that simultaneously deliver high cell voltages and energy density in combination with good cyclability and stability.
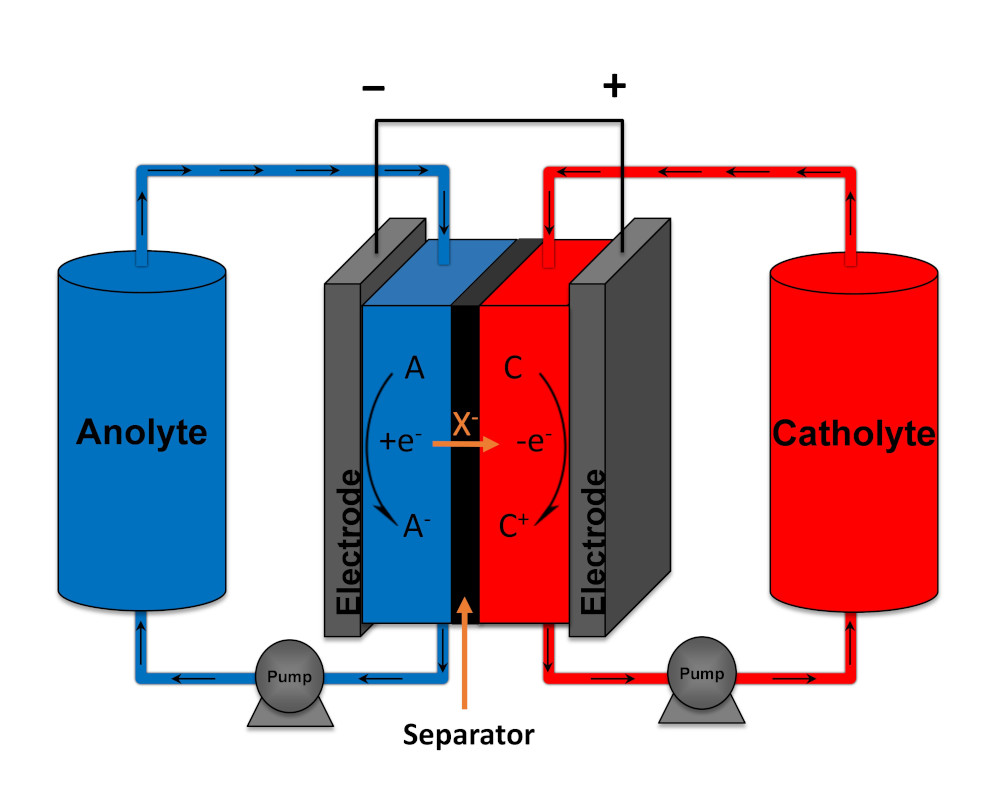
Principe of a redox flow battery
Within M2N we are working on organic flow batteries in close collaboration with the Dutch Institute for Fundamental Energy Research (DIFFER):
Organic molecules for energy storage in redox flow batteries
We develop new molecules that are expected to meet the stringent requirements for this type of energy storage and advance the state-of-the-art of this technology. To discover materials that simultaneously fulfill all desired parameters such as a high redox potential, excellent solubility, and excellent (cycling) stability, we use a unique interdisciplinary approach that combines organic synthesis, electrochemistry and application in prototype devices. In addition we work with a computational group at DIFFER to elucidate new structure-property relations and predict new compounds by DFT modelling.
Membrane evaluation
Apart from the redox active species in a RFB, the membrane separating the two half-cells is one of the crucial components in the device. Typically this element has the highest electrical resistance of the whole system and therefore has a large impact on the energy efficiency of the battery, especially at high charging or discharging rates. Its role is twofold as it must keep the anolyte and catholyte molecules separated while being highly conductive towards the supporting electrolyte. Additionally, it must have sufficient mechanical stability to withstand the shear forces of the electrolyte flow and resist swelling from the solvent. Membranes that combine all of these properties are therefore very scarce. Together with collaborators, the M2N group is developing and testing tailored membranes for this application.
Prototype flow batteries
To fully evaluate the performance of new redox active materials and membranes, a large focus lies on studying flow battery prototypes in the battery lab at DIFFER. These devices allow us to quickly evaluate the cycling stability and energy efficiencies of selected material combinations and point out what parameters need to be worked on, both on the engineering side as well as molecular design. Thanks to the straightforward scalability of flow batteries, these small prototype devices pave the way towards real world implementation of RFB storage technology.
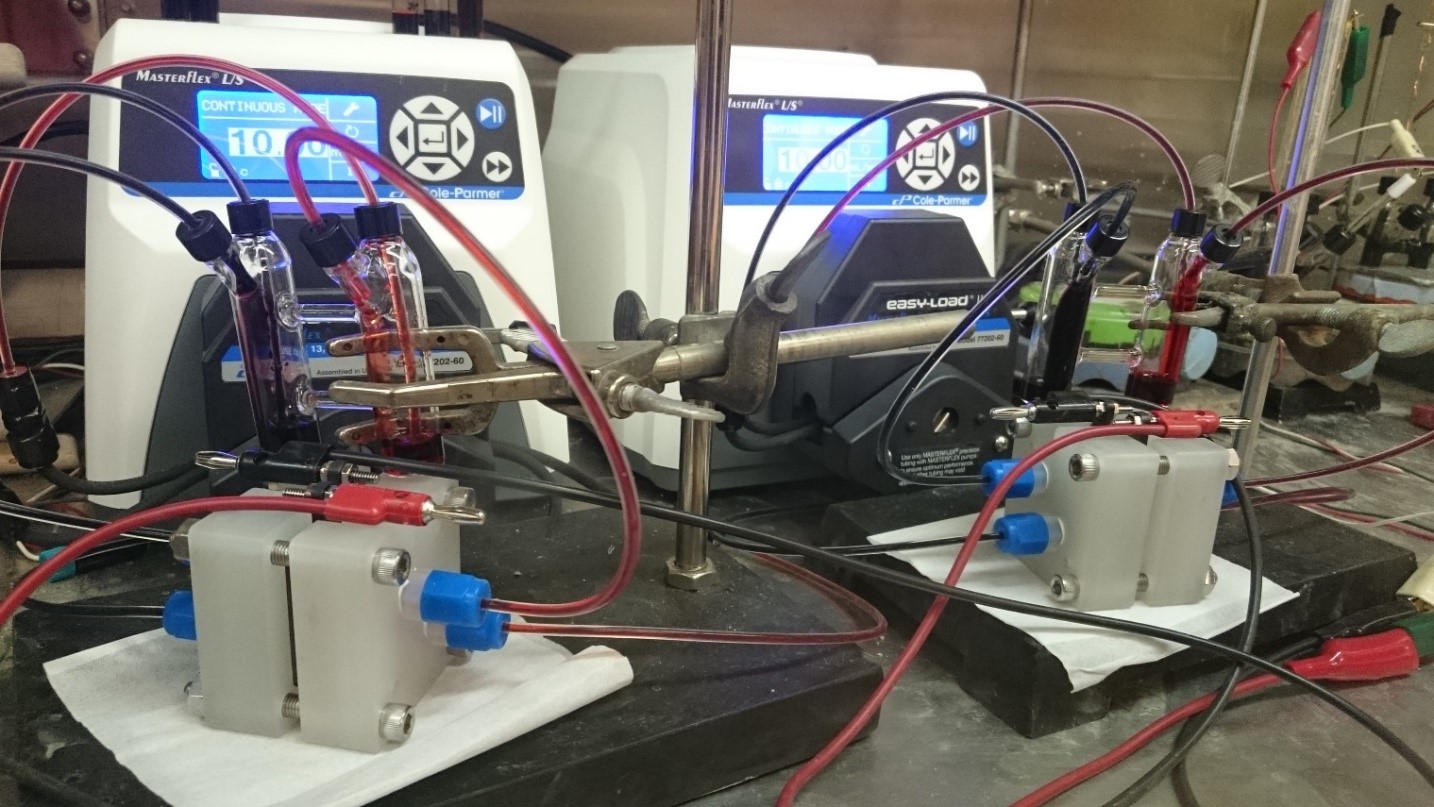
Redox flow batteries ion operation
Contact
Koen Hendriks
René Janssen